Alkanolamine
In organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl (−OH) and amino (−NH2, −NHR, and −NR2) functional groups on an alkane backbone. Most alkanolamines are colorless.[1] Alkanolamine's bifunctionality and physicochemical characteristics lead to its use in many applications, such as textiles, cosmetics, agricultural chemical intermediates, drugs, and metal working fluids.[2][3]
- Alkanolamines
-
Methanolamine, from the reaction of ammonia with formaldehyde
-
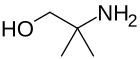
2-amino-2-methyl-1-propanol is a precursor to oxazolines
-
Sphingosine is a component of some cell membrane.
1-Aminoalcohols
[edit]1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member. 1-Aminoalcohols tend to be labile, readily converting to more highly condensed derivatives or hydrolyzing to the amine and carbonyl.
2-Aminoalcohols
[edit]2-Aminoalcohols are an important class of organic compounds that are often generated by the reaction of amines with epoxides:
- C2H4O + R−NH2 → RNHC2H4OH
Simple alkanolamines are used as solvents, synthetic intermediates, and high-boiling bases.[1]
Hydrogenation or hydride reduction of amino acids gives the corresponding 2-aminoalcohols. Examples include prolinol (from proline), valinol (from valine), tyrosinol (from tyrosine).
Key members: ethanolamine, dimethylethanolamine, N-methylethanolamine, Aminomethyl propanol. Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinally useful derivative of ethanolamine.[citation needed]
1,3-, 1,4-, and 1,5-amino alcohols
[edit]- Heptaminol, a cardiac stimulant
- Propanolamines
Natural products
[edit]Most proteins and peptides contain both alcohols and amino groups. Two amino acids are alkanolamines, formally speaking: serine and hydroxyproline.
- Veratridine and veratrine
- Tropane alkaloids such as atropine
- hormones and neurotransmitters epinephrine (adrenaline) and norepinephrine (noradrenaline)
References
[edit]- ^ a b Martin Ernst; Johann-Peter Melder; Franz Ingo Berger; Christian Koch (2022). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001.pub2. ISBN 978-3-527-30673-2.
- ^ Davis, John W.; Carpenter, Constance L. (1997), Ware, George W. (ed.), "Environmental Assessment of the Alkanolamines", Reviews of Environmental Contamination and Toxicology, vol. 149, New York, NY: Springer New York, pp. 87–137, doi:10.1007/978-1-4612-2272-9_2, ISBN 978-1-4612-7482-7, retrieved 2025-03-19
- ^ Laskar, Ranjini; Dutta, Subhabrata; Spies, Jan C.; Mukherjee, Poulami; Rentería-Gómez, Ángel; Thielemann, Rebecca E.; Daniliuc, Constantin G.; Gutierrez, Osvaldo; Glorius, Frank (2024-04-17). "γ-Amino Alcohols via Energy Transfer Enabled Brook Rearrangement". Journal of the American Chemical Society. 146 (15): 10899–10907. doi:10.1021/jacs.4c01667. ISSN 0002-7863. PMC 11027157. PMID 38569596.
External links
[edit]- Amino+Alcohols at the U.S. National Library of Medicine Medical Subject Headings (MeSH)